Nominal voltage: 3.2V
Nominal capacity: 4500-6500mAh
Application: instrumentation, backup power source, special equipment
Nominal voltage: 3.2V
Nominal capacity: 4500-6500mAh
Application: instrumentation, backup power source, special equipment
Nominal voltage: 3.6V
Nominal capacity: 3000-4800mAh
Application: digital devices, power tools
Nominal voltage: 3.7V
Nominal capacity: 2000-3500mAh
Application: special equipment, medical equipment, robot, etc.
Charge temperature:-20℃ ~ +55℃
Discharge temperature:-40℃ ~ +60℃
Application:sepcial equipment,special,polar science
Nominal voltage:3.2V
Nominal capacity:500mAh
Application:Internet of Things locator card
Nominal voltage:12.0V
Nominal capacity:12000mAh
Battery cell:26650/3.2V/3.2Ah
Nominal voltage:25.6V
Nominal capacity:40000mAh
Battery cell:148F20C/3.2V/20Ah
Nominal voltage:48.0V
Nominal capacity:40000mAh
Battery cell:26650/3200mAh/3.2V
Lithium iron phosphate battery refers to the lithium ion battery using lithium iron phosphate as the positive electrode material. Lithium iron phosphate battery is considered as a new generation of lithium ion battery because of its advantages such as high safety, long cycle life, rate discharge and high temperature resistance.
Large Power can provide customers with cell, BMS (power management system), integrated structure of the battery customized solutions, so as to meet the customers personalized power needs.
Power storage, special equipment, robot, AGV, rail transit, medical equipment, emergency backup, electric communication, etc.








Due to the stability and reliable safety design of the positive electrode materials, the lithium iron phosphate battery pack has been passed rigorous safety tests, and will not explode even in violent collisions.
The 1C cycle life of lithium iron phosphate battery generally reaches 2000 times, even more than 3500 times. The energy storage market requires more than 4000-5000 times, which is higher than other types of lithium batteries.
The peak heat of lithium iron phosphate battery can reach 350~500℃. And it has wide working temperature range (-20~+75℃). Even under high temperature (60℃), it can still give off 100% capacity.
The battery can be fully charged by a dedicated charger after 40 minutes of 1.5C charging.
Lithium iron phosphate batteries are environmentally friendly, non-toxic, pollution-free, and cheap. It also has wide availability of raw materials.
The nominal voltage of the single lithium iron phosphate battery is 3.2V, the charging voltage is 3.6V, and the discharge cut-off voltage is 2.0V.
Lithium iron phosphate battery packs reach the required voltage by the equipment through battery cell series connection. The battery voltage is equal to N* series connection number. Common lithium iron phosphate battery voltages are as follows:
The capacity of lithium iron phosphate battery pack is determined by the capacity and number of the battery cells connected in parallel, generally according to the specific requirements of electrical equipment. The more lithium iron phosphate battery cells connected in parallel, the larger the capacity is.
Common lithium iron phosphate battery capacity has 10 ah, ah, ah, 40, 50, ah, ah, 100 200 ah, ah 400, etc.
As shown in the figure, the left part is the olivine structure LiFePO4, the positive electrode of the battery. The aluminum foil is connected to the battery positive electrode and then polymer separator separates the positive and negative electrode, so that Li + and e - cannot pass the separator. The right part is battery cathode composed of carbon (graphite). The copper foil is connected to the battery cathode.
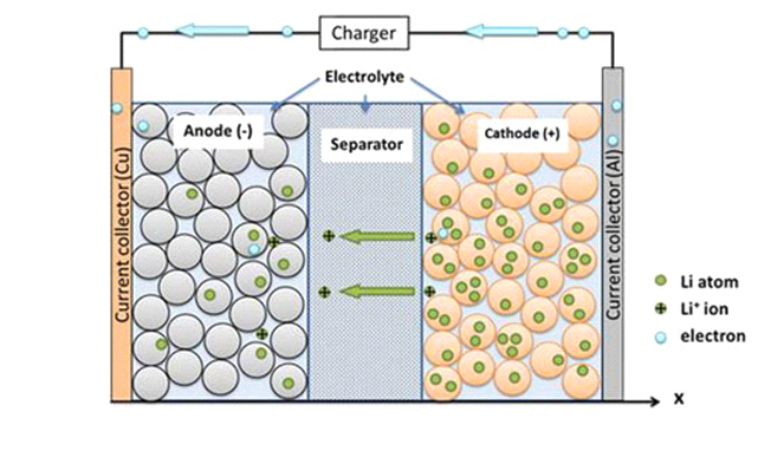
When the LiFePO4 battery is charging, Li+ in the positive electrode migrates to the negative electrode through the polymer separator. In the discharge process, Li+ in the negative electrode migrates to the positive electrode through the separator. Lithium-ion batteries are named for the way lithium ions move back and forth during charge and discharge process.
The CCCV charging method is recommended for lithium iron phosphate battery pack, that is, constant current first and then constant voltage. Constant current recommendation is 0.3c, while constant voltage recommendation is 3.65V.
Solar panels cannot directly charge the lithium iron phosphate battery, because the voltage of the solar panel is unstable. It needs voltage regulation circuit and corresponding lithium iron phosphate battery charging circuit.
The generator cannot directly charge the lithium iron phosphate battery, because the electricity generated by the generator is alternating current or pulsed direct current. The lithium iron phosphate battery must be charged by direct current with constant voltage.
| Chemistry | Voltag (V) | Energy Density (wh/kg) | Working Temp (℃) | Cycle Life | Safety | Environmenta | Cost based on cycle life x wh of SLA |
| LiFePO4 | 3.2 | >120 | -20-60 | >2000 | Safe | Good | 0.15-0.25 lower than SLA |
| Lead acid | 2.0 | >35 | -20-40 | >200 | Safe | Not Good | 1 |
| NiCd | 1.2 | >40 | -20-50 | >1000 | Safe | Bad | 0.7 |
| NiMH | 1.2 | >80 | -20-50 | >500 | Safe | Good | 1.2-1.4 |
| LiMnxNiyCoz02 | 3.7 | >160 | -20-50 | >500 | better than LiCo | OK | 1.5-2.0 |
| LiCoO2 | 3.7 | >200 | -20-50 | >500 | Unsafe w/o PCM | OK | 1.5-2.0 |
Affected by the structure, lithium iron phosphate and Ternary battery have their own advantages and disadvantages in performance. Ternary battery has advantages in energy density and fast charging speed, while lithium iron phosphate battery has advantages in cycle life, safety and economy.
The cathode, electrolyte and separator are similar in both types of batteries, but the biggest difference is the positive electrode material, hence the name.
| Anode Material | LiFePO4 | LiNixCoyMn1-x-yO2 |
| Shorthand | LFP | NCM |
| Nominal Voltage | 3.2V | 3.65V |
| Crystal Form | Olivine Structure | Layer Structure |
| Lithium ion extraction channel | One Dimension | Two Dimensions |
As for the cell, ternary battery has higher energy density. The rated voltage and theoretical specific capacity (mAh/g) of lithium iron phosphate anode materials are all lower than those of ternary batteries, and their energy density has been the best.
| Anode Material | Rated Voltage (V) | Theoretical Specific Capacity (mAh/g) | Estimated Actual Specific Capacity(mAh/g) | Estimated Operating Cell Energy Density(wh/kg) |
| LiFePO4 | ~3.2 | ~170 | ~145 | ~170 |
| NCM811 | ~3.65 | ~274 | ~195 | ~240 |
| NCM523 | ~170 | ~210 | ||
| NCM111 | ~145 | ~180 |
Note: The energy density of the cell should be evaluated in combination with the cell design and process. The table value is for reference only.
Ternary lithium batteries have a great advantage over lithium iron phosphate batteries in charging efficiency.
When the ternary lithium battery and lithium iron phosphate battery are charged below 10C, there is no significant difference in the constant current ratio. When the charging ratio is above 10C, the constant current ratio of the lithium iron phosphate battery will decrease rapidly, and the charging efficiency will decrease rapidly.
Theoretically, lithium iron phosphate has advantages in cycle life. The olivine structure is more stable, is not easy to swell and has more stable electrochemical reaction.
Lithium iron phosphate batteries have incomparable advantages in safety. The positive electrode voltage is low, and there is no oxygen-releasing heat chain reaction that ternary has. The thermal stability temperature can reach above 300℃, while that of ternary battery is around 150-200℃.
Lithium iron phosphate LiFePO4 has obvious advantages on price at present, the raw materials are relatively cheap, and the domestic industry chain is relatively mature.
Cobalt is the key to lowering the price of NCM batteries. Cobalt is mainly an associated mineral with low production and uneven distribution, and its price has been rising continuously in recent years.
Lithium iron phosphate batteries tend to have more than 2000 cycles in this case; Small lithium battery manufacturers with lower quality battery also have over 1000 cycles;
Most of the applications of high rate discharge batteries are power-type lithium ion batteries, and most of them are used to provide power to the motor. As most lithium iron phosphate batteries operate under high load, the decay time of battery materials is accelerated, and the cycle life is around 800 times.
The lithium iron phosphate batteries used in this case have a shorter lifespan, which is only about 300 times.
The high temperature performance of lithium iron phosphate battery is not very mature at present. The operating temperature ranges from -20℃ to 125℃, which is the theoretical value, and the practical application temperature range is smaller.
Lithium iron phosphate batteries tend to have more than 2000 cycles in this case; Small lithium battery manufacturers with lower quality battery also have over 1000 cycles;
Most of the applications of high rate discharge batteries are power-type lithium ion batteries, and most of them are used to provide power to the motor. As most lithium iron phosphate batteries operate under high load, the decay time of battery materials is accelerated, and the cycle life is around 800 times.
The lithium iron phosphate batteries used in this case have a shorter lifespan, which is only about 300 times.
Low temperature has a larger impact on the performance of lithium iron phosphate batteries. According to the current market situation, the service life of lithium iron phosphate batteries operating below -20℃ to -40℃ is significantly reduced, and it is around 300 times.
When choosing a charger, it is better to use a charger with the correct device to cut off, so as not to shorten the service life of lithium iron phosphate batteries due to overcharging. In general, a slow charge will prolong the battery life, which is better than a quick charge.
The discharge depth is the main factor affecting the life of lithium iron phosphate batteries. The higher the discharge depth is, the shorter the life of lithium iron phosphate batteries will be. In other words, by reducing the discharge depth, the life of lithium iron phosphate batteries can be significantly extended. Therefore, we should avoid over discharge lithium battery UPS to extremely low voltage.
If lithium iron phosphate battery is used at high temperature for a long time, its electrode activity will decrease and its service life will be shortened. Therefore, it is a good way to extend the life of lithium iron phosphate battery by keeping the operating temperature as appropriate as possible.
Decommissioned lithium iron phosphate batteries which do not have the value of cascade utilization and the batteries after cascade utilization will eventually enter the stage of disassembly and recycling. Unlike ternary material batteries, lithium iron phosphate batteries do not contain heavy metals, and the recovery products are mainly Li, P and Fe. The additional value of the recovery products is low, so low-cost recovery methods are important. There are mainly fire metallurgy and hydrometallurgy technical processes.
Conventional fire metallurgy recovery is generally a high-temperature incineration of electrode, in which carbon and organic matter in the electrode fragments are burned off, and the remaining ash that cannot be burned off is finally filtrated to obtain fines containing metals and metal oxides.
The hydrometallurgy recovery method is mainly to dissolve the metal ions in the lithium iron phosphate battery through acid-base solutions, and extract the dissolved metal ions in the form of oxides and salts through precipitation and adsorption. In the reaction process, H2SO4, NaOH, H2O2 and other reagents are often used.