Brief description of high performance quasi-solid-state room temperature sodium-sulfur battery
Jul 12, 2019 Pageview:1340
Compared with traditional sodium-sulfur batteries (operating temperature 300~350 °C), room temperature sodium-sulfur batteries have significant advantages such as high energy density (1274Whkg-1) and good safety. However, current room temperature sodium-sulfur batteries still face many problems, such as severe self-discharge caused by the polysulfide shuttle effect, short cycle life, and dendrite growth caused by sodium metal anodes. The physical sulfur fixation method which conventionally combines sulfur with a porous carbon material does not sufficiently suppress the shuttle effect. Therefore, the introduction of chemical bonds in the sulfur-carbon composites to enhance the sulfur fixation effect is a reasonable idea for the development of high-performance sulfur electrodes. On the other hand, the use of polymer electrolytes instead of organic electrolytes can significantly reduce the safety hazards of battery ignition and liquid leakage. However, polymer electrolytes generally have low conductivity and high-impedance electrode/electrolyte interface defects, limiting their sodium, application in sulfur batteries.
In view of this, recently, the research group of Professor Wang Guoxiu of the University of Science and Technology of Sydney, the research group of Professor Li Baohua of the Shenzhen Graduate School of Tsinghua University, and the research group of Prof. Michel Armand of the CICEnergigune Institute of Spain collaborated to develop high-performance polymer sulfur cathode materials and gels by organic synthesis. The polymer electrolyte material is applied to a room temperature sodium-sulfur battery to exhibit excellent cycle performance and safety. Through mechanism analysis, it is found that the polymer sulfur electrode can effectively fix sulfur through chemical bonds to inhibit the shuttle effect; at the same time, the high conductivity gel polymer electrolyte not only can significantly inhibit the diffusion of polysulfide, but also help to form stable sodium in the cycle. Metal anode/polymer electrolyte interface. The article was published in the international top chemical journal Angew.Chem.Int.Ed. (Impact factor: 12.102).
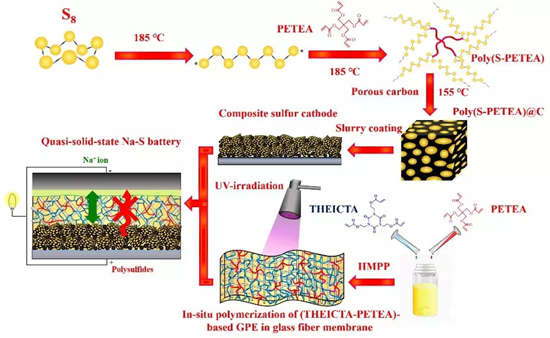
Figure1. Schematic diagram of the preparation of a quasi-solid sodium-sulfur battery, first, a polymer sulfur material was prepared by adding pentaerythritol tetraacrylate (PETEA) monomer to molten sulfur at 185 ° C, and then compounded with a porous carbon matrix to obtain a polymer sulfur@carbon cathode material. Subsequently, PETEA monomer and tris(2-acryloyloxyethyl) isocyanurate (THEICTA) monomer were dissolved in an organic electrolyte, and a flexible gel polymerization was prepared in situ in a glass fiber membrane under UV irradiation electrolyte membrane. Finally, a quasi-solid-state room temperature sodium-sulfur battery was assembled with a composite polymer sulfur electrode, a gel polymer electrolyte and a sodium metal anode.
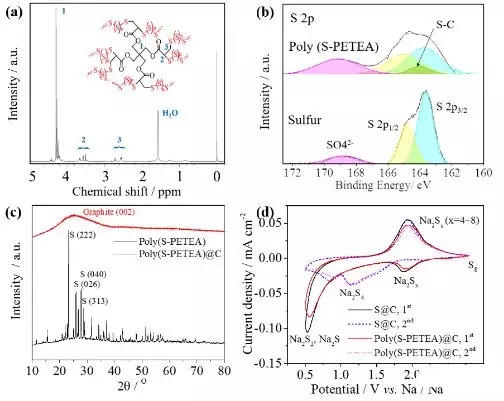
Figure2. Characterization of a polymer sulfur electrode (a) 1H-NMR and (b) S2pXPS spectra; (c) XRD spectrum of polymer sulfur and polymer sulfur@carbon; (d) sodium/electrolyte/sulfur@carbon and sodium/electrolyte/polymer sulfur@ The CV curve of the carbon battery at 0.1 mV/s.
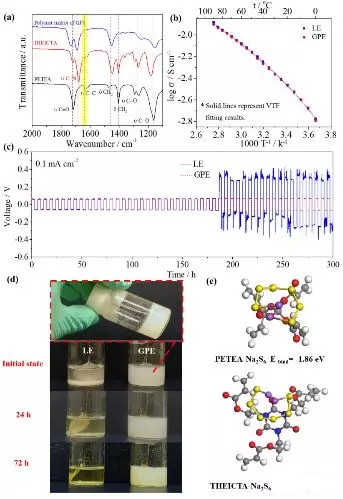
Figure3. Characterization of a (PETEA-THEICTA) based gel polymer electrolyte (a) Infrared spectral changes before and after gel formation; (b) Conductivity-temperature change curve. The gel polymer electrolyte has a room temperature conductivity of 3.85×10-3 S/cm; (c) a constant current circulation curve of a sodium/gel polymer electrolyte/sodium symmetrical battery at 0.1 mA/cm 2 ; (d) an electrolyte and Visual observation of polysulfide formation/diffusion in gel polymer electrolytes; (e) Binding energy between polysulfide Na2S6 and PETEA and THEICTA.
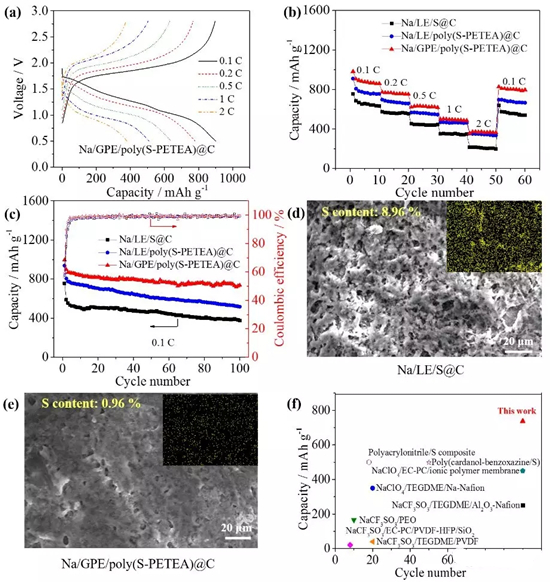
Figure4. Electrochemical performance of a quasi-solid sodium-sulfur battery: (a) charge and discharge curves at different currents and (b) rate performance; (c) battery cycle performance at 0.1C. It can still reach 736 mAh/g after 100 cycles. (d) Sodium/electrolyte/sulfur@carbon and (e) sodium/gel polymer electrolyte/polymer sulfur@carbon battery The morphology of the sodium metal negative electrode after 100 cycles of 0.1 C. The use of a gel polymer electrolyte significantly inhibits polysulfide deposition and sodium dendrite growth; (f) performance comparison with reported polymer room temperature sodium sulfur batteries.
Summary:
1) A novel polymer sulfur electrode and a functionalized gel polymer electrolyte material were successfully prepared by using a star-crosslinking monomer;
2) In quasi-solid-state room temperature sodium-sulfur batteries, the polymer sulfur electrode achieves strong sulfur fixation through chemical bonds, while the gel polymer electrolyte with high conductivity and high safety can simultaneously inhibit polysulfide diffusion and stabilize sodium metal. Negative Electrode/Electrolyte Interface; this dual optimization allows the room temperature sodium-sulfur battery to exhibit good reversible capacity and cycle performance.
This work has opened up a new path for the development of low-cost, high-performance room-temperature sodium-sulfur batteries.
The page contains the contents of the machine translation.
- Prev Article: What kind of material is the electrode of the fuel cell?
- Next Article: What are the “new desserts” in energy storage technology?
Leave Message
Hottest Categories
-
Hottest Industry News
-
Latest Industry News









