The effect of ambient humidity on the nickel anode material of lithium battery was investigated
Aug 14, 2019 Pageview:1122
The technology of high specific energy lithium ion power battery has been considered by the industry. However, high nickel positive electrode is faced with many problems, among which the preservation of raw materials and high requirements of battery production environment are huge challenges. This paper briefly summarizes the environmental factors, especially the influence of humidity on the characteristics of high nickel anode materials.
For nickel-based materials, spontaneous reactions will occur on the surface of particles, Ni3+ will be converted into Ni2+, and O2- will be released. When materials with high nickel content (NMC622, NMC811, NCA, etc.) are exposed to air, they are more likely to absorb carbon dioxide and water in the air, and the following reactions will occur:
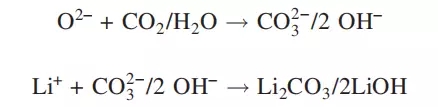
n this way, Li2CO3 and LiOH layers are formed on the particle surface. The higher the Ni ratio in the material, the higher the PH value will be. However, Li2CO3 and LiOH consume Li in the material and have no electrochemical activity, which will cause capacity attenuation. LiOH also reacts with PVDF and LiPF6, adversely affecting the process and performance of the battery.
The reaction between materials and air will be carried out in the whole process of raw material preservation, electrode preparation, electrode sheet storage, etc. Therefore, for high nickel materials, strict environmental control is required from raw materials to the whole battery production process, especially water control. If moisture and reactions have taken place in the material, through the conventional drying process can't again to remove the influence of moisture, the preparation of electrode paste, pole piece manufacturing all need to be done in the dry environment, in general, the positive battery production process requires high nickel dew point - 30 ℃ environment.
If the high nickel anode material particles absorb moisture from the air and react to produce LiOH, this will have a serious impact on the manufacturing process of the anode plate. In the preparation process of high-nickel positive paste, PVDF is dissolved in NMP, and the basic groups on the surface of the material will attack the adjacent c-f and c-h bonds. PVDF is easy to undergo bismolecular elimination reaction, and a part of the carbon-carbon double bond will be formed on the molecular chain. The reaction is as follows:
hen the double bond in PVDF is increased, the adhesive force will also increase, which will lead to the paste viscosity increase, and even the paste will form a gel state. As a result, high nickel anode paste in the preparation and the coating process, the influence of environmental humidity is huge, if the water absorption reaction in the process, especially easy to cause the slurry properties change, cause pole piece manufacturing process quality is not stable, poor process consistency problem, forming gel slurry, cannot even coating process.
In addition, when the bonding force is increased due to the double bond increase in PVDF, the brittle chip increase is particularly prone to fracture, and the chip fracture causes the process process to be unable to be carried out in the process of sheet rolling, slitting and other processes. If the battery is a square winding process, at the corner of the winding core, the pole sheet will fracture or drop material.
LiOH reacts with Al foil as follows:
OH - 6 + 2, + 6 h2o - OH - + 2 al (OH) 3 + 3 h2
After the corrosion of Al, the mechanical strength decreases, the electrochemical properties and safety of the battery will be affected, and the change of the corrosion surface properties of the foil, the coating peeling strength will be reduced, the mechanical and electrical properties of the sheet will be affected.
In addition, LiOH also reacts with LiPF6, consuming Li ions in the electrolyte and generating HF gas, which can corrode the metal parts inside the battery and cause the battery to leak eventually. Moreover, HF damages SEI membrane, which will continuously react with the major components of SEI membrane:
ROCO2Li + HF - ROCO2H + LiF
LiCO3 + 2 + 2 and hf H2CO3 lif
Finally, LiF precipitates are generated inside the battery to cause irreversible chemical reactions of lithium ions in the negative electrode plate of the battery, and the energy of the battery is reduced when the active lithium ions are consumed.
The high nickel absorbing water reaction product Li2CO3 is easy to decompose into CO2 gas under the high potential of charging state, resulting in the battery bulge and leakage problem. When the water absorbed by the material is enough, the gas produced will be more, and the pressure inside the battery will become larger, which will cause the battery to be stressed out, resulting in the battery swelling, leakage and other dangers.
Therefore, for high nickel anode materials, the environmental humidity needs to be strictly controlled in the process of raw material preservation and battery preparation in order to produce high-performance lithium ion batteries.
The page contains the contents of the machine translation.
Leave Message
Hottest Categories
-
Hottest Industry News
-
Latest Industry News









