-
Chemistry and Construction+
- Lithium ion battery
- Lead acid battery
-
Energy Density
-
Cycle Life
-
Discharge Rate
-
Discharge Voltage
-
Charging Characteristics
-
Temperature Influence
-
Application and Use
-
Maintenance
Lead Acid Battery VS Lithium Ion Battery: Complete Comparison
Sep 23, 2024 Pageview:2231
Let's explore the difference between lithium and lead acid battery. Lead-acid batteries and lithium batteries are very common backup power, in choosing which battery is more suitable for your device application, due to the different characteristics of the two batteries, you need to take into account a number of factors, such as voltage, capacity, number of cycles and so on.
Chemistry and Construction
Lithium ion battery
Lithium-ion batteries operate based on reversible electrochemical reactions between two electrodes (anode and cathode) immersed in an electrolyte. Lithium-ion Battery uses lithium compounds in the electrodes (typically lithium cobalt oxide, lithium iron phosphate, etc.) and a non-aqueous electrolyte (organic solvent with lithium salts). The electrodes are typically thin layers on a conductive substrate, allowing for a high energy density.
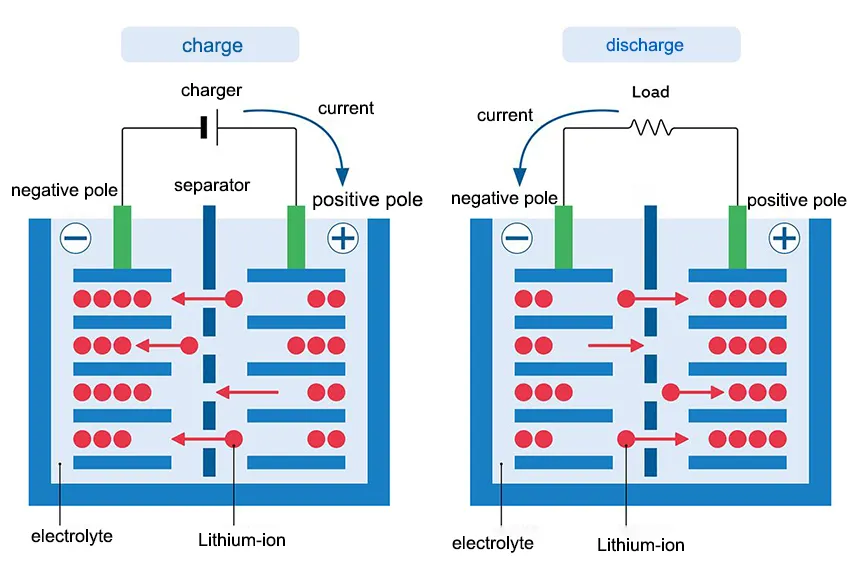
Negative Electrode typically made of graphite, the anode in a Li-ion battery hosts lithium ions (Li⁺) when the battery is charged (during discharge, lithium ions move from anode to cathode). Positive Electrode typically made of graphite, the anode in a Li-ion battery hosts lithium ions (Li⁺) when the battery is charged (during discharge, lithium ions move from anode to cathode).
During charging, lithium ions move from the cathode through the electrolyte and intercalate (embed) into the anode material. When discharging, the process reverses: lithium ions move from the anode to the cathode through the electrolyte. While lithium ions migrate through the electrolyte, electrons flow through an external circuit, generating an electric current that can power devices.
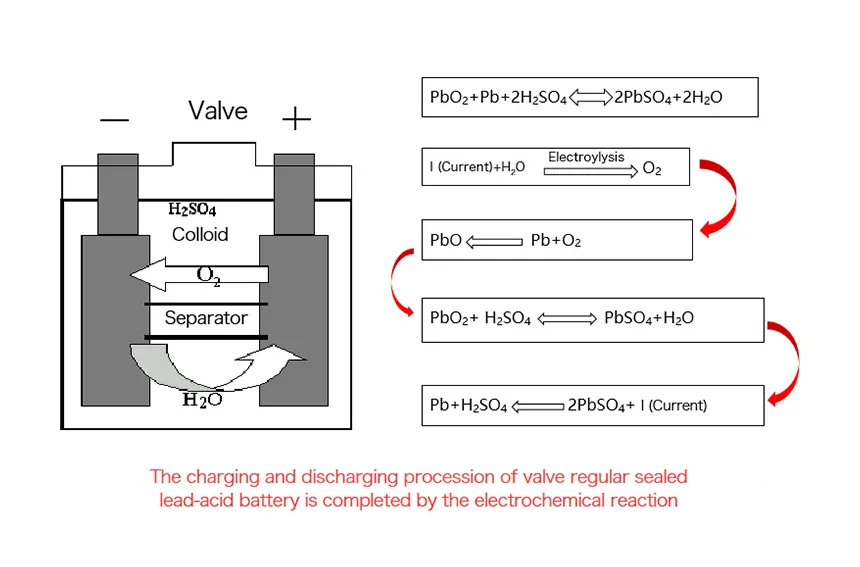
Lead acid battery
The working principle of a lead-acid battery involves electrochemical reactions between lead and lead dioxide electrodes immersed in a sulfuric acid electrolyte, providing a reliable source of electrical energy. The electrodes are thick and heavy due to the nature of the lead-based chemistry.
Energy Density
Lithium-ion Battery generally has a higher energy density compared to lead-acid batteries. This means it can store more energy per unit of volume or weight, making it lighter and more compact for a given energy capacity.
Lithium-ion batteries typically have a significantly higher volume energy density compared to lead-acid batteries. This means Li-ion batteries can store more energy per unit of volume, allowing for smaller and more compact battery packs.
Lead-acid Battery has a lower energy density compared to lithium-ion batteries, which results in a larger and heavier battery for the same energy storage capacity.
Similarly, Li-ion batteries have a higher weight energy density compared to lead-acid batteries. This results in lighter battery packs for a given energy capacity, which is crucial for applications where weight is a critical factor, such as in electric vehicles.
Cycle Life
The cycle life of a battery refers to the number of charge-discharge cycles it can undergo before its capacity degrades to a specified level.
Lithium-ion Battery typically has a longer cycle life compared to lead-acid batteries. They can endure hundreds to thousands of charge-discharge cycles, depending on the specific chemistry and usage conditions.
Lead-acid Battery while robust, lead-acid batteries generally have a shorter cycle life compared to lithium-ion batteries, especially if subjected to deep discharges.
Li-ion batteries are favored in applications requiring longer cycle life, higher energy density, and lighter weight, such as in electric vehicles and portable electronics, energy storage. Lead-acid batteries remain competitive in applications where cost-effectiveness, reliability, and established recycling infrastructure are critical factors, such as in automotive starting batteries and stationary power backup systems.
Discharge Rate
The discharge rate of a battery refers to how quickly it can release its stored energy, often measured in terms of C-rate.
Li-ion batteries are capable of high discharge rates, typically ranging from 1C to 2C or higher depending on the specific chemistry and design. Li-ion batteries maintain relatively stable voltage and capacity even at high discharge rates, which is crucial for applications requiring bursts of power or sustained high-power output.
Lead-acid batteries are generally not designed for high discharge rates compared to Li-ion batteries. Common discharge rates for lead-acid batteries range from 0.05C to 0.2C, depending on the specific type (flooded, AGM, or gel). Some AGM (Absorbent Glass Mat) or high-performance lead-acid batteries can handle moderate discharge rates up to 0.5C or slightly higher.
Lead-acid batteries may experience voltage sag and reduced capacity when subjected to high discharge rates, the discharge rate of lithium is stable, and the lead acid is gradually lost to 60%. This limitation makes them less suitable for applications requiring rapid energy release or high power demands.
Lead-acid batteries are better suited for applications where moderate discharge rates are sufficient, such as in automotive starting batteries, backup power systems, and stationary applications. They are less effective in applications needing rapid discharge due to voltage drop and reduced efficiency at higher rates.
Lithium-ion batteries are superior in terms of high discharge rates compared to lead-acid batteries. They can provide significant power output quickly and efficiently, making them ideal for applications like electric vehicles, power tools, and certain industrial equipment that require high-power bursts.
Discharge Voltage
Lithium-ion batteries maintain a relatively stable voltage throughout most of their discharge cycle, providing consistent power output until near the end of their capacity. This makes them suitable for applications requiring stable voltage and consistent performance over the discharge period.
Lead-acid batteries have a lower nominal voltage per cell compared to lithium-ion batteries. They exhibit a more gradual decline in voltage during discharge, with a more pronounced drop towards the end of their capacity. This characteristic is typical for applications where the battery’s voltage range and discharge profile are critical considerations.
In practical applications, understanding these voltage characteristics helps in selecting the appropriate battery type based on the voltage requirements, discharge profile needs, and overall performance expectations.
Charging Characteristics
Lithium-ion Battery can be charged quickly, and some types of Li-ion batteries can handle rapid charging without significant damage. They also have lower self-discharge rates compared to lead-acid batteries.
Lead-acid Battery typically charges more slowly than lithium-ion batteries, especially when nearing full capacity. Rapid charging can cause heat build-up and may require careful monitoring.
For example, a 3000 mAh (milliampere-hour) Li-ion battery with a charging current of 1500 mA (1.5A) would theoretically take around 2 hours to charge from empty to full (assuming 1C charging rate). A 12-volt lead-acid battery with a capacity of 100 Ah (ampere-hours) and charging at a rate of 10 amps (which is about 0.1C) would take approximately 10 hours to charge from empty to full.
Temperature Influence
Li-ion batteries perform best within a moderate temperature range typically between 0°C to 45°C (32°F to 113°F). Lead-acid batteries are more tolerant of temperature extremes compared to Li-ion batteries. They can operate effectively within a range of -20°C to 50°C (-4°F to 122°F).
Lithium-ion batteries are more sensitive to temperature extremes compared to lead-acid batteries. They require careful thermal management to ensure safe and optimal performance. Cold temperatures (below 0°C) can decrease the battery’s capacity and power output temporarily. Extremely low temperatures can cause the battery to become sluggish and may lead to irreversible damage if the battery is charged or discharged at very low temperatures.
Lead-acid batteries have a wider operating temperature range and can withstand higher and lower temperatures better than Li-ion batteries. While lead-acid batteries can tolerate higher temperatures better than Li-ion batteries, excessive heat can still accelerate battery aging and increase water loss from flooded batteries.
Both battery types require monitoring and adherence to temperature guidelines to ensure safe operation and longevity.
Application and Use
the higher energy density of lithium-ion batteries contributes to their widespread adoption in portable electronics, electric vehicles, and energy storage systems where maximizing energy per unit volume or weight is essential.
Lithium-ion Battery commonly used in portable electronics (laptops, smartphones), electric vehicles (EVs), and renewable energy storage systems due to their high energy density and lighter weight.
Lead-acid Battery traditionally used in automotive starting batteries, backup power systems (UPS), and industrial applications (forklifts, golf carts) due to their durability, low cost, and suitability for high current outputs. Lead-acid Battery while lower in energy density, remain competitive in applications where cost-effectiveness, robustness, and established infrastructure for recycling and disposal are prioritized over weight and size considerations.
Maintenance
Lithium-ion Battery generally requires less maintenance compared to lead-acid batteries. They do not require electrolyte checks or periodic equalization charges. Lithium-ion batteries are generally easier to install due to their lighter weight, compact size, and flexible mounting options. They require less structural support and may have fewer ventilation requirements compared to lead-acid batteries.
Lead-acid Battery requires periodic maintenance such as checking electrolyte levels, specific gravity, and ensuring proper charging to prevent sulfation. Lead-acid batteries require sturdy mounting structures and adequate ventilation due to their heavier weight and gas emissions during charging. They also require specific maintenance access for periodic checks and servicing.
In conclusion, the choice between Lithium-ion and Lead-acid batteries depends on specific application needs, including power requirements, space constraints, cost considerations, and environmental factors. Each battery type has distinct advantages that make it suitable for different industries and applications.
- Prev Article: Lithium Batteries in Cold Weather – Everything You Need to Know
- Next Article: Sodium ion battery VS Lithium ion battery
Leave Message
Hottest Categories
-
Hottest Industry News
-
Latest Industry News









